USF Health set to start COVID-19 vaccine trial on children in fall
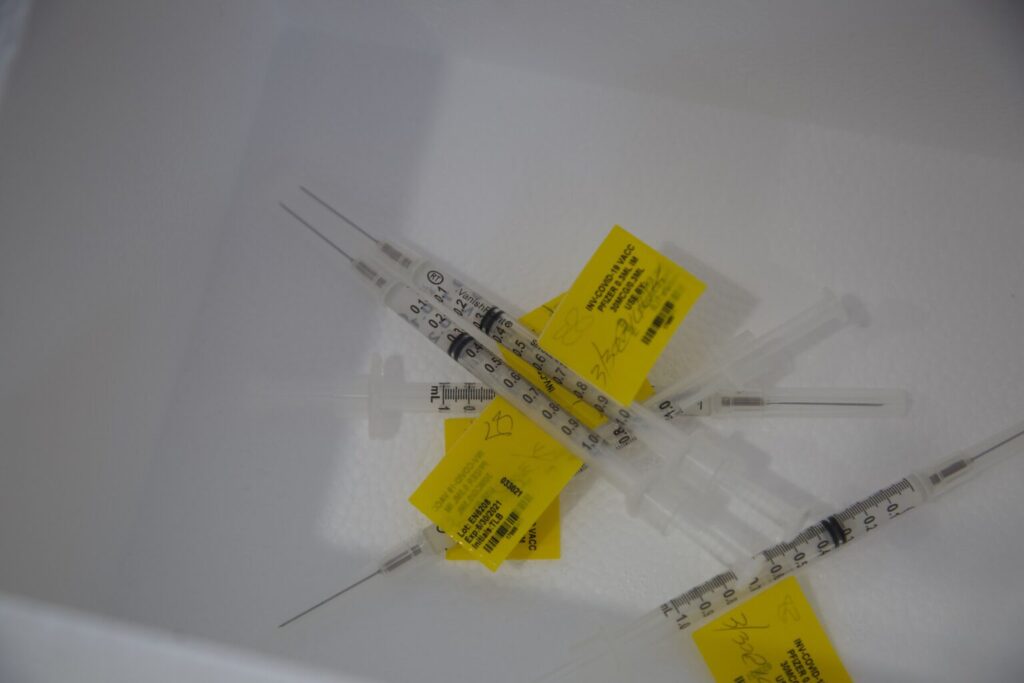
The USF Health Morsani College of Medicine has been selected by Moderna as one of the approximately 80 sites in the U.S. to hold clinical trials testing the effects of the Moderna vaccine on children.
Elizabeth Enriquez-Bruce, director of research for the Department of Pediatrics, said around 100-120 children will participate in USF’s trial of the estimated 12,000 children nationwide. The trials will take place throughout the fall, depending on which age group a child classifies under.
“We are enrolling the first cohort of 6 years old to 12 years old currently,” she said. “Then, any child between the ages of 2 to 5 years will be enrolled in mid September, and the final age group of 6 months to under 2 years of age will take place in mid October.”
Families will also receive a $100 gift card as an incentive for participating in the trial. Enriquez-Bruce also said an additional $50 will be given out for completing the e-diary for each participant, which will monitor a participant’s temperature, physical well-being and any other noticeable symptoms or concerns about their health.
The e-diary, she said, must be completed daily throughout the duration of the study, which could last for 12 months.
Children must be anywhere between ages 6 months to under 12 years and in an overall healthy and stable condition to participate in the trial, Carina Rodriguez, principal investigator overseeing the trial and chief of the division of pediatric infectious diseases, said.
“This is a placebo-controlled randomized trial. So three children receive the active vaccine versus one receiving the placebo,” she said. “It will be a two-dose vaccine separated by 28 days. After the vaccination process is complete, they will be followed and monitored after the second dose for 12 months.”
Rodriguez said the volunteers will visit the department five to six times throughout the trial. They’ll go in for the two doses, and then visit for checkups after two, six and 12 months. If any concerns arise, the children can be brought in for full evaluations.
USF was selected as a trial site by Moderna due to the university’s experience with previous vaccination trials, according to Rodriguez. She hopes the trial will grow the availability of the vaccine for all ages, and further combat the effects of the pandemic on the world.
“It’s really bringing the possibility of having a common vaccine for the pediatric population,” Rodriguez said. “Bringing this vaccine to the younger population will expand protection and immunity in children, so we get the greater scenario in how we’re dealing with a surge due to children being back in school and being vulnerable to exposure. Usually the vaccine will minimize the result of the child bringing this infection to their home.”
Enriquez-Bruce said the trial could show the efficacy and safety of the vaccine in younger children.
“The ultimate goal of this trial is to use this data for approval of the vaccine in this age group,” she said. “Overall, we are making sure the vaccine is safe for younger children, so therefore the children will develop an immune reaction that will protect them against the coronavirus.”
While Rodriguez said it’ll be difficult to predict when concrete results will be ready, she said the current efforts are moving along smoothly and have received praise from the community.
“This particular trial will enroll over 12,000 children in the U.S., and based on the data, [Moderna] will decide when they will present the information to the [Food and Drug Administration],” she said.
“So I cannot really speculate at this point on timeline, but I know the trial is really moving along very nicely, and in about 10 days we have almost 50% of the recruitment activity done. It’s pretty amazing that the response from the community has been really overwhelming.”
Though the results of the trial are the main focus, Rodriguez said the highlight of the trial has been hearing an enthusiastic response from the community through forming connections with the participants and their families.
“We have had a lot of families very interested in participating, and it has been really amazing to chat with the children and ask them questions such as ‘Why are you here today,’ ‘What are you expecting?’ and ‘Why do you want to participate,’” she said.
“I think there is so much of this kind of desire to help people moving forward, as we’re all in this battle against COVID together. It’s not that there is no one solution, but certainly the vaccine is a big one, and if we can accomplish it for all the different ages, that’ll be life changing.”